Important Safety Information
Contraindication: Repatha® is contraindicated in patients with a history of a serious hypersensitivity reaction to evolocumab or any of the excipients in Repatha®. Serious hypersensitivity reactions including angioedema have occurred in patients treated with Repatha®.
Hypersensitivity Reactions: Hypersensitivity reactions, including angioedema, have been reported in
patients treated with Repatha®. If signs or symptoms of serious hypersensitivity reactions occur,
discontinue treatment with Repatha®, treat according to the standard of care, and monitor until signs
and symptoms resolve.
Adverse Reactions in Primary Hypercholesterolemia: The most common adverse reactions (>5% of patients treated with Repatha® and more frequently than placebo) were: nasopharyngitis, upper respiratory tract infection, influenza, back pain, and injection site reactions.
From a pool of the 52-week trial and seven 12-week trials: Local injection site reactions occurred in 3.2% and 3.0% of Repatha®-treated and placebo-treated patients, respectively. The most common injection site reactions were erythema, pain, and bruising. Hypersensitivity reactions occurred in 5.1% and 4.7% of Repatha®-treated and placebo-treated patients, respectively. The most common hypersensitivity reactions were rash (1.0% versus 0.5% for Repatha® and placebo, respectively), eczema (0.4% versus 0.2%), erythema (0.4% versus 0.2%), and urticaria (0.4% versus 0.1%).
Adverse Reactions in the FOURIER Cardiovascular Outcomes Trial: The most common adverse reactions (>5% of patients treated with Repatha® and more frequently than placebo) were: diabetes mellitus (8.8% Repatha®, 8.2% placebo), nasopharyngitis (7.8% Repatha®, 7.4% placebo), and upper respiratory tract infection (5.1% Repatha®, 4.8% placebo).
Among the 16,676 patients without diabetes mellitus at baseline, the incidence of new-onset diabetes mellitus during the trial was 8.1% in patients treated with Repatha® compared with 7.7% in patients that received placebo.
Immunogenicity: Repatha® is a human monoclonal antibody. As with all therapeutic proteins, there is potential for immunogenicity with Repatha®.
Indications
Repatha® is indicated:
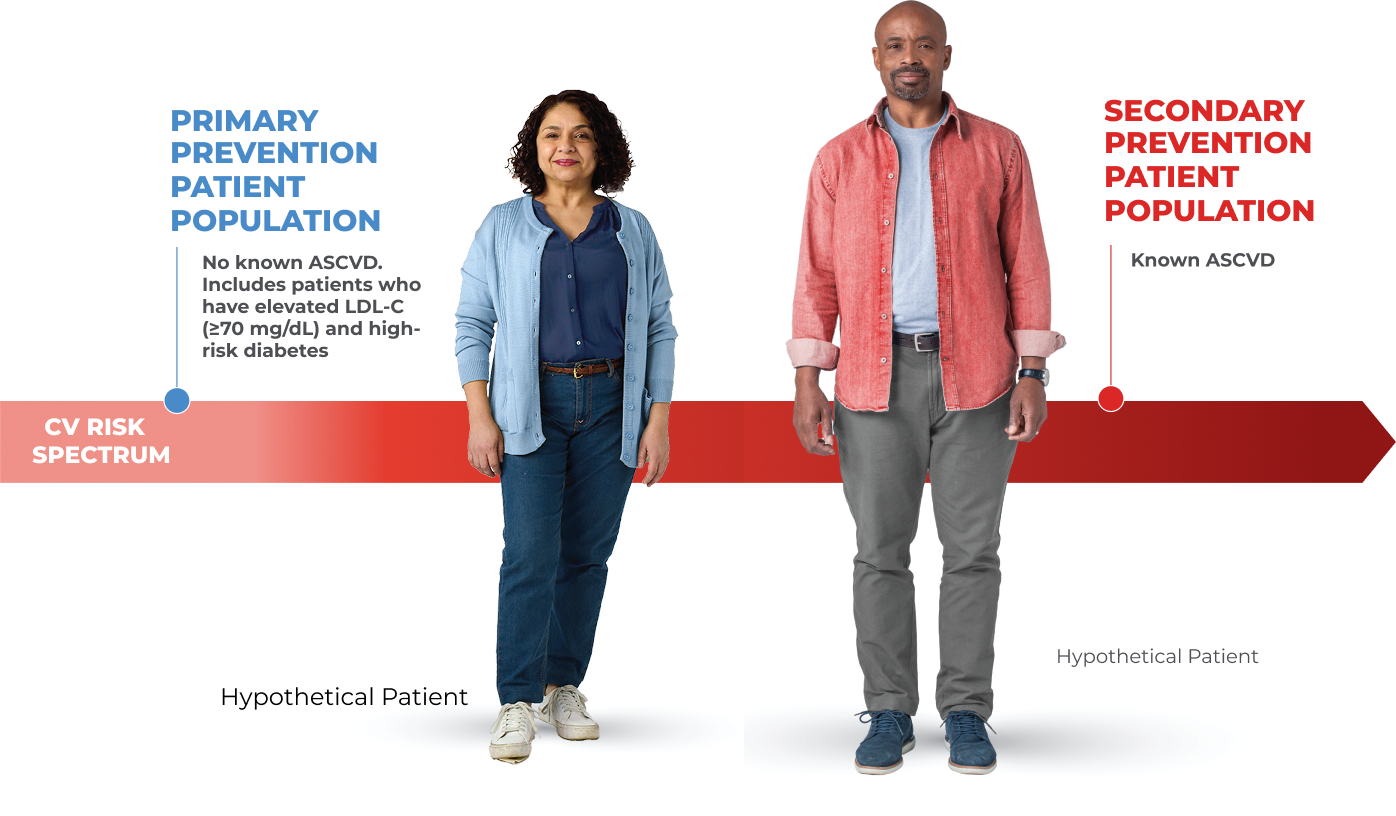
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, myocardial infarction, stroke, unstable angina requiring hospitalization, or coronary revascularization) in adults at increased risk for these events.
- As an adjunct to diet and exercise to reduce low-density lipoprotein cholesterol (LDL-C) in adults with hypercholesterolemia and in adults with heterozygous familial hypercholesterolemia (HeFH)