INDICATIONS
Repatha® is indicated:
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, myocardial infarction, stroke, unstable angina requiring ...
Repatha® is indicated:
Please direct your patients to Amgen® SupportPlus: A dedicated transition support line through 844-Repatha , prompt 2 to assist with Q&A, process, and new Repatha® prescriptions.
Hours: Monday-Friday
Amgen has made the decision to discontinue the Repatha® prefilled syringe.
This decision was made to uphold the high standards Amgen has for patient experience. We have seen unprecedented demand for Repatha® globally and in the United States. We are focused on meeting the growing demand for Repatha® that we expect will continue, while delivering the best experience for adults at increased risk for major adverse CV events (MACE) and hypercholesterolemia.
As part of our long-term planning, we did an evaluation of the device presentations in the US. Today there are 2 device presentations:
We plan to discontinue the Repatha® prefilled syringe by Q1 2026. All patients should transition to the SureClick® autoinjector not made with natural rubber latex and require a new prescription.
Transitioning Patients to a New Device with a Prescription
There are no concerns about the Repatha® drug product, efficacy, or patient safety. Alternative presentations are available, and Amgen has sufficient product to meet the demand of patients transitioning from the prefilled syringe.
HCP Action Required:
The Repatha® SureClick® autoinjector is available and will be appropriate for most patients. However, they will require a new prescription to transition.
Prefilled single-dose syringe
Discontinued
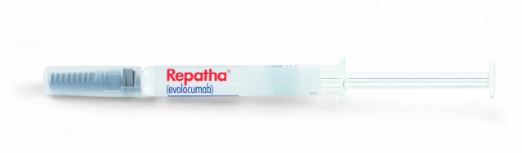
The Repatha® prefilled single-dose
syringe will be discontinued
effective Q1 2026.
140 mg/mL prefilled
single-dose syringe
(NDC 72511-501-01)
SureClick®autoinjector-
Active and Available
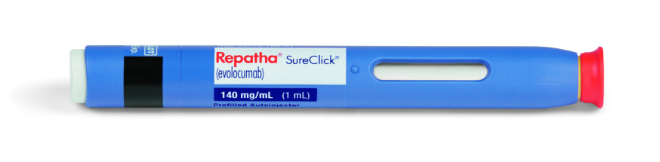
The Repatha®SureClick® autoinjector will be patients. is required.
Repatha® 140 mg/mL
prefilled single-dose
SureClick® autoinjector
(NDC 72511-393-02)
Amgen is committed to supporting existing patients’ transition and has several dedicated services in place, including:
Additional resources to help facilitate the transition for your patients:
You and your team might want to know more about the process and the rationale behind this decision. Please review the FAQs document for more information.
We appreciate your support and collaboration as we make this transition.
If you need more information, don't hesitate to contact Amgen MedInfo at
medicalinformation@amgen.com
Sincerely,
Amgen Inc.